Abstract
Introduction Current concepts in pediatric and adult immune thrombocytopenia (ITP) have resulted in treatment guidelines that include the clinically relevant differences between these groups. However, defining ITP in only two age categories-children and adults-is inappropriate because it oversimplifies the clinical characteristics and needs of adolescents and young adults (AYAS). In a previous analysis we have reported the high risk of chronic disease at 12 months (50%), as well as factors associated with chronicity (e.g. initial platelet count; initial therapy). Little is known about the long-term course of chronic ITP and treatment strategies in AYAS. This study aims to describe clinical and laboratory data of AYAS with chronic primary ITP until 48 months follow-up (FU). This is the first step to better predict outcome and design age-adapted therapies.
Methods Data were extracted from the PARC-ITP, CARMEN-France and OBS'CEREVANCE registries, open since 2004, 2013, and 2004, respectively. PARC-ITP is an international multi-center registry collecting data prospectively of children and adults with newly diagnosed ITP; CARMEN-France enrolls all incident ITP in adults ≥18 years from many French centers, and OBS'CEREVANCE is a national cohort of children <18 years with cytopenias including chronic ITP. Patients aged 12-25 years with primary chronic ITP at 12 months were included. Chronic disease was defined as platelet count <100 × 109/l or ongoing treatment at 12 months or relapse at later FU. Remission beyond 12 months was defined as platelet count >100 × 109/l without need of treatment for at least 6 months; "late remission" in the period >12-36 months, and "very late remission" between 36-48 months. Patients with a diagnosis of secondary ITP before 12 months (n=70), misdiagnosed ITP (n=4) at any timepoint and pregnant women (n=12) were excluded. Descriptive statistics were used to analyze data.
Results A total of 428 AYAS (64% female) with chronic primary ITP were included. Mean age at study enclosure was 15 (SD 2·7) and median platelet count 50 × 109/L (IQR 26;81). FU information was available for 88% at 24 months, 77% at 36 months and 59% at 48 months, with 266, 98 and 64 patients classified with sustained chronic disease at last available FU (≥24 months), remission after 12 months, and unknown remission status, respectively. The initial median platelet count was 15 × 109/l (IQR 7;35) and 74 patients (19%) reported no bleeding at diagnosis. Overall, 7 patients (1.6%) reported intracranial hemorrhage, 3 at initial diagnosis, 3 in the first 6 months and 1 between 12 and 24 months.
Patients with sustained chronic disease had median platelet count of 55 and 62 × 109/L at 24 and 48 months FU, respectively. Around 50% needed platelet-enhancing treatment over FUs. Percentage of bleeders in this subgroup and bleeding location was similar across all FU, with 70 % suffering of wet bleeding.
Despite the increasing proportion of patients receiving 2nd/3rd line therapies across FUs, intravenous immunoglobulins (IVIG) and corticosteroids were still reported for about 40% and 30% of treated patients over all FUs.
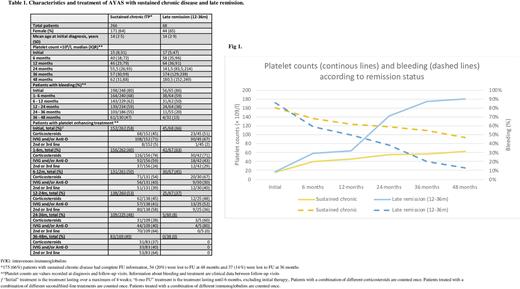
Remission beyond 12 months FU was diagnosed in 98 cases (23%). The number of new remissions was constant over FU (38 (10%), 30 (9%) and 30 (12%) patients at 24, 36 and 48 months FU, respectively). Initial clinical characteristics were similar for patients with "late remission", "very late remission" or sustained chronic disease. However, AYAS with "late remission" displayed less bleeding, higher platelet counts and received less IVIG at 6 and 12 months than AYAS with sustained chronic disease (Table 1, Fig 1). This was not the case for patients with "very late remission".
In total, 11 patients were diagnosed with secondary ITP after 12 months: 2 common variable immunodeficiency, 4 Evans syndrome and 5 patients with systemic lupus erythematosus.
Conclusion In this first study of long-term FU of AYAS with chronic primary ITP, it revealed that a substantial percentage of patients will achieve treatment-free remission after 12 months. AYAS with "late remission" had a less severe disease course in the first year of disease (excluding initial presentation) than those with sustained chronic disease. For patients with sustained chronic disease at last FU the bleeding phenotype exhibits strong stability over time, so as the number of patients receiving corticosteroids or IVIG, despite increased use of 2nd/3rd line therapies.
Disclosures
Schifferli:Takeda: Honoraria; Sobi: Honoraria; Novartis: Honoraria, Research Funding. Moulis:Sanofi: Research Funding; CSL Behring: Research Funding; Grifols: Other: Meeting attendance grants, Research Funding; Novartis: Other: Meeting attendance grants, Research Funding; Amgen: Other: Meeting attendance grants, Research Funding. Godeau:Sobi: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions; Grifols: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions. Michel:UCB: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions; argenx: Other: Boards, speaker at educational sessions; Sobi: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions. Kuehne:Amgen: Honoraria, Research Funding; Sobi: Honoraria; UCB: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal